IDMP-O
Identification of Medicinal Product (IDMP)
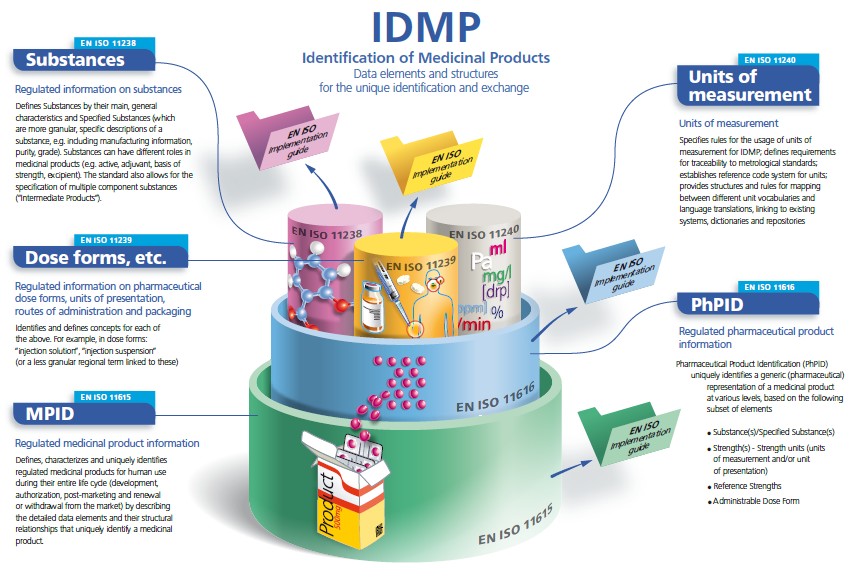
Initially published by the ISO organization (https://www.iso.org/news/ref2234.html), IDMP encompasses five ISO documents:
- ISO 11238 about Substances
- ISO 11240 about Units of Measurement
- ISO 11616 about Pharmaceutical Product Identification
- ISO 11615 about Medicinal Product Identification
- ISO 11239 about Dose Forms, Units of Presentation, Routes of Administration and Packaging
Problem statement
Even with the ISO IDMP providing definitions for human understanding, challenges remained regarding inconsistent data representation and communication barriers among IT systems managing IDMP data.
This absence of standardization obstructs effective data exchange between systems, which in turn affects collaboration among humans. As a result, it impacts the efficiency of clinical testing and the reporting of new drugs to health authorities.
Objective
Create a machine-readable and machine-actionalbe digital format of ISO IDMP enabling data sharing and integration across different platforms and organizations.
